What is iron ore
Iron ore is a mineral aggregate that contains iron element or iron compound and can be used economically. It can be used for refining elemental pig iron, steelmaking and many other purposes.
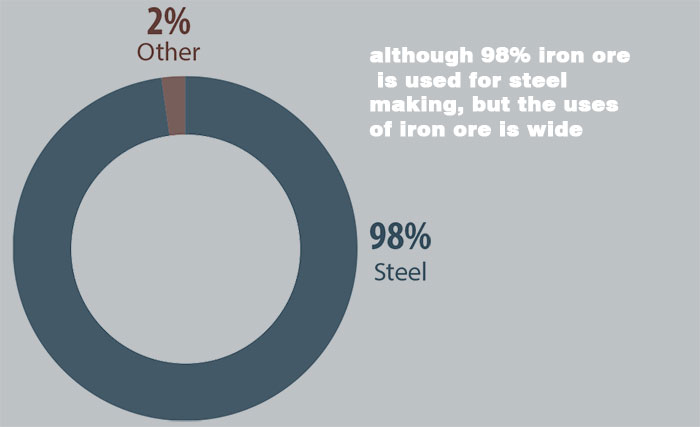
About 98% of iron ore is used to produce iron, it is one of the important sources used to refine steel. In steel mills, natural ore (iron ore) undergoes procedures such as crushing, grinding, magnetic separation, flotation, and gravity separation to gradually select enriched iron ore powder.
Iron content is one of the most important factors for iron ore to be used for steelmaking. Use the enriched iron ore powder to produce elemental iron through reduction in various ironmaking furnaces (nomal furnaces like BF, shaft furnace, rotary kiln, etc.). Primary ore can also be used, but the efficiency sometimes is relatively low. The fine iron ore will be pelletized or briquetted into balls and entered into the steel manufacturing process. The elemental iron is smelted into various steels with different carbon content and various element contents through various steelmaking furnaces (such as EAF, BOF, induction furnace, etc.).
Pig iron is divided into steelmaking pig iron, foundry pig iron and alloy pig iron according to its usage. Steel is divided into carbon steel and alloy steel according to its constituent elements. Alloy steel is based on carbon steel, in order to improve or obtain certain properties, an appropriate amount of one or more elements are added. There are many types of elements added, mainly Cr, Mn, V, Ti, Ni, Mo, Si, etc.
Although the vast majority of iron ore is used to make steel, it has many other applications.
e.g.
- Iron ore is also used as a catalyst for the synthesis of ammonia (pure magnetite)
- Natural mineral pigments (hematite, speculiarite, limonite)
- Feed additives (magnetite, hematite, limonite)
- Precious medicine stone (magnet), etc., very small amout
Various iron ores have different uses due to their composition and texture. Common iron ores include Magnetite, Hematite, Goethite, Limonite, Siderite, Pyrite, Wustite, etc.
| Name | Formula | wt % Fe |
|---|---|---|
| Magnetite | Fe3O4 | 72.36 |
| Hematite | Fe2O3 | 69.94 |
| Goethite | FeO(OH) | 62.58 |
| Limonite | FeO(OH) · n(H2O) | 52 (n = 1) |
| Siderite | FeCO3 | 48.2 |
| Pyrite | FeS2 | 46.5 |
| | ||
| Wustite | FeO | 79 |
| The better known iron minerals | ||
Magnetite
Chemical composition: FeFe2O4
Relative density: 4.8-5.3 g/cm3
Hardness (Mosh): 5-6
Color: Iron black, or dark ingot with black stripes.

Due to its strong magnetism, magnetite can be attracted by permanent magnets, indicating that it is magnetic. It is with an iron content of 72.4% and is one of the most common iron ores.
It is formed, endogenous and metamorphic. It is found in magma or iron deposits. Contact metasomatism iron deposits, gasification high-temperature rare earth iron deposits, sedimentary metamorphic iron deposits, and a series of iron deposits or iron ores related to volcanism are the main mineral components and important minerals as ironmaking raw material.
Uses of magnetite iron ore
- It is used primarily in the production of iron and steel.
- It is used in the Haber-Bosch and Fischer-Tropsch processes as a catalyst, for the production of ammonia and hydrocarbons respectively; and as a tool in the degradation of pollutants from industrial processes.
- In medicine, ferrofluids of magnetite have been studied for the treatment of hypothermia. Other applications have been shown in hyperthermic therapies, in MRI contrast agents and in DNA extraction techniques. The Chinese medicine vital honey pellets are made of magnetite, good for eyesight.
- Magnetite has also been used in recording media, as a pigment material and for water purification of iron removal, manganese removal, fluorine removal, etc.
Hematite
Chemical formula: Fe2O3
Relative density: 5-5.3 g/cm3
Hardness (Mohs): 5.5-6
Color: Apparently crystalline hematite is iron black or steel gray, aphanitic is dark red, streaked cherry red

The name hematite comes from ancient Greek, meaning like blood, mainly because of its color. As early as 63 BC, a Babylonian writer mentioned that King Methrizetus had many hematites in his private gem collection, and at the time hematite was believed to be a potent talisman.
Hematite is an iron mineral that is widely distributed in nature. It can be formed in various geological processes, but it is mainly hydrothermal, sedimentation, and sedimentary metamorphism. It is another important iron ore for ironmaking.
Uses of hematite iron ore
- One of the main iron ores for iron and steelmaking, hematite can be used as raw material for blast furnace ironmaking according to different particle size, grade and impurity content
- Hematite is an excellent inorganic anti-rust pigment, so it has important industrial uses to make paint
- pigments with hematite
- Feed additives
- Additives for cosmetics
- Laser flash powder
- Filler for plastic products (e.g. smart phone cases)
- Manufacture soft and hard magnetic materials
- Hematite iron concentrate can be directly used to produce welding rods, to produce steel-making cooling nets, chemical pigments, and to produce storage batteries
- A traditional Chinese medicine – haematitum (Pacify Liver and subdue yang, check adverse rise of qi by heavy settling, cool blood and stop bleeding)
Goethite
Chemical formula: FeO(OH)
Relative density: 4.28g/cm3, soil type can reach 3.3 g/cm3
Hardness (Moshs): 5-5.5
Color: Black, brown to stone-coloured, almost transparent to opaque, diamond to earthy luster

The great German writer Goethe discovered goethite, but at the beginning, because this mineral has no fixed form and is difficult to distinguish, people have always classified it as limonite. Until 1806, people named this iron mineral after Goethe in memory of him.
Goethite is rich in iron and is usually formed when other iron-rich minerals such as pyrite combine with oxygen and break down through weathering. Goethite is often associated with minerals such as calcite and quartz, which together form a hard crust on the weathered minerals. Goethite of sedimentary origin is found in lakes and springs. Goethite in hydrothermal deposits is formed at low temperature and is symbiotic with quartz, siderite, etc. During regional metamorphism, iron hydrates were dehydrated to goethite.
Goethite is the “rainbow brother” of the iron oxide family which can be iridescent with a multicolored rainbow-like display. Cause it can coagulate on sandstone in thin layers of a similar size to the wavelengths of light, and the refraction and reflection of the layers produce a beautiful iridescent optical effect that shimmers as the piece is turned in the light.
Uses of goethite iron ore
- Its main modern use is as an iron ore
- Goethite is an important component of ochre pigments
- Fine goethite specimens are rare and therefore are valued collectibles
- Elimination of cadmium which is the chemical element from the contaminated waters in industrial liquid wastes
Limonite/Brown iron ore
Chemical formula: FeO(OH)*nH2O4
Relative density: 2.7-4.3 g/cm3
Hardness (Moshs): Hardness varies depending on its composition and form. Silicon-rich dense lumps can have a hardness of 5.5. Mud-rich earthy ones can have a hardness of 1
Color: Brown in various shades, streaked tan, semi-metallic and earthy

Limonite actually does not refer to a single mineral, but is mainly composed of iron hydroxides such as goethite and ferric goethite, and contains a mixture of hydrous silica and mud, so the composition changes greatly. It is the weathering product of various iron-bearing minerals, especially the surface part of the metal sulfide deposit. After the mine is oxidized, it often forms limonite.
- Skarn limonite is mainly composed of limonite, hematite and quartz
- High silicon limonite is mainly composed of limonite, hematite, goethite and quartz
Limonite was previously thought to be an independent mineral with a composition of 2Fe2O3*3H2O, but X-ray diffraction analysis showed that most of them were aphanitic goethite, which could be mixed with lepidocite, hematite, quartz, clay etc., containing adsorbed water and capillary water, the composition is variable, but basically it is FeO(OH)·nH2O. Physical properties also vary, but are always various shades of brown, streaked tan. It is usually called stalactite, grape shape, dense and loose massive, earthy and other outputs, and often appears in the false appearance of pyrite crystal form.
Limonite is a very common secondary substance under oxidative conditions, which is formed by the oxidation of iron sulfide or carbonate. It is very common in the oxidation zone of sulfide deposits, forming an “iron cap” and can be used as a sign of ore prospecting. Rust is also composed of limonite. Limonite can also be formed by inorganic or biological precipitation in swamp, lake and spring deposits.
Uses of limonite iron ore
- Ironmaking. Although the iron content of limonite is lower than magnetite and hematite, it is relatively loose and easy to smelt, make it also another important iron ore in steel plant
- Used as a pigment, it is the pigment substance in loess
- As a mineral medicinal material in traditional Chinese medicine – Yu Yu Liang (for the treatment of chronic diarrhea, dysentery, hemorrhage in stool, metrorrhagia and vaginal discharge)
Pyrite
Chemical formula: FeS2
Relative density: 4.95-5.2 g/cm3
Hardness (Moshs): 6-6.5
Color: Lead brass color, surface often has tint, greenish black stripes, opaque

Named in antiquity from the Greek “pyr” for “fire”, because sparks flew from it when struck with another mineral or metal. Known to Dioscorides (~50 CE) under the name “περι υληζ ιατρικηζ” which included both pyrite and chalcopyrite. Because of its light brassy color and bright metallic luster, it is often mistaken for gold, so it is also called “fool’s gold”.
Pyrite includes pyrite, pyrrhotite and marcasite. Pyrite is common, pyrrhotite is less common with very weak magnetism, and marcasite is rare.
The pyrite family
| Name | Chemical formula | Crystal | Color | Stripe | Luster | Hardness | Density (g/cm3) |
| pyrite | FeS2 | Lsometric | Golden yellow, brass | Green black | Metallic | 6-6.5 | 4.95-5.2 |
| pyrrhotite | FenSn+1 | Hexagon | Dark bronze yellow | Grey black | Metallic | 4 | 4.58-4.7 |
| marcasite | FeS2 | Rhomboid | Pale brassy yellow | Dark gray green | Metallic | 5-6 | 4.6-4.9 |
Pyrite is the most widely distributed sulfide in the earth’s crust, formed in a variety of different geological conditions. Generated in copper-nickel sulfide magmatic deposits, characterized by being rich in Ni. Generated in contact metasomatized deposits, often containing Co. Generated in polymetallic hydrothermal deposits.
Pyrite components include Cu, Zn, Pb, Ag, etc. The content has increased. In the deposits related to volcanism, the content of As and Se in the pyrite composition has increased. The pyrite of exogenetic origin is found in sedimentary rocks, sedimentary deposits and coal seams, and often forms nodules and agglomerates.
Under surface oxidation conditions, pyrite is easily decomposed to form various iron sulfates and hydroxides. Among iron sulfates, jarosite is the most common. Among iron hydroxides, goethite is the most common, which is the main mineral component of limonite. Limonite sometimes appears as a false pyrite (ie, limonite in the form of cubes, which is the crystalline form of pyrite).
Uses of pyrite iron ore
- Produce sulfuric acid, the slag can be used to make iron and steel. If the slag contains high sulfur content and low iron content, it can be used as an auxiliary raw material for cement
- For chemical raw materials
- Production of sulfur and various sulfur-containing compounds to be used in industries like rubber, papermaking, textiles, food, and matches. Also can be used in agriculture industry as well as in the manufacture of various explosives, smoke agents, etc.
- When it coexists with copper, lead, zinc, molybdenum and other sulfide deposits, and contains gold, cobalt, molybdenum and rare element selenium, it can be comprehensively recycled






