The Main Source of Ammonium Chloride
The combined Alkali process (Hou’s process) is an advanced method used for the production of soda ash. Ammonium chloride (NH4Cl) is the main by-product of this process, with 1 ton of ammonium chloride produced for every 1 ton of soda ash. As the global production of soda ash via the combined Alkali process increases annually, the output of ammonium chloride also continues to rise.
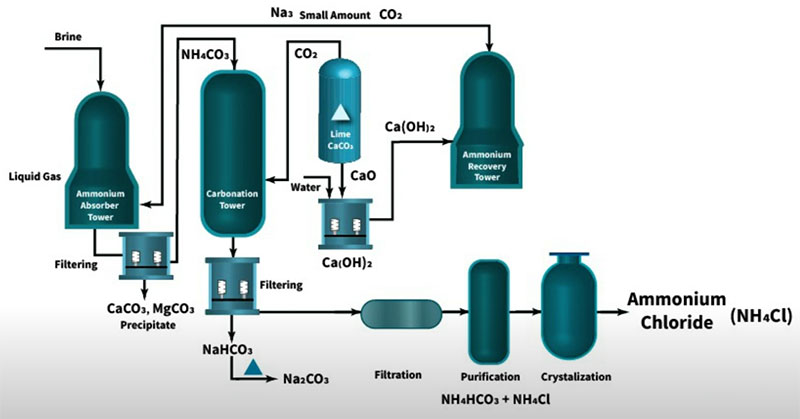
Ammonium chloride, as a form of ammonium salt, can be converted into other ammonium salts with higher utilization value through various reactions. However, there are no large-scale industrial applications of ammonium chloride conversion technology reported internationally. The main reasons are:
- Ammonium chloride is a relatively stable salt with stable physical and chemical properties, making conversion techniques limited and difficult to apply on an industrial scale.
- The conversion technology and process conditions for ammonium chloride are stringent, resulting in high production costs.
Apart from industrial applications in dry batteries, accumulators, ammonium salts, tanning, electroplating, precision casting, pharmaceuticals, photography, electrodes, adhesives, yeast culture, and more, ammonium chloride’s primary application in agriculture is to provide nitrogen nutrients in compound fertilizers.
The Ammonium Chloride Fertilizer
Ammonium chloride is a physiological acidic quick-acting nitrogen fertilizer with a nitrogen content of 22% to 25%. It has a fast fertilizer effect, a long duration, can accelerate photosynthesis, promote crop metabolism, enhance the synthesis of chlorophyll in plants, and increase crop resistance to diseases, pests, and lodging.
Ammonium chloride is suitable for neutral and calcareous soils and can play a significant role in soil improvement and pH regulation of soil solutions. It can be directly applied to crops such as wheat, rice, corn, and rapeseed, and is particularly effective for fiber crops like cotton and hemp, enhancing fiber toughness and tensile strength, thereby improving quality. The fertilizer effect of ammonium chloride in paddy fields is significant, as chloride ions inhibit nitrifying bacteria, reducing nitrogen loss. Chloride ions can be washed away with water, preventing excessive ion residue in the soil.
However, ammonium chloride should not be used on chlorine-sensitive crops like tobacco, sugarcane, sugar beets, tea trees, potatoes, watermelon, and grapes, nor should it be used in arid regions or poorly drained saline-alkali soils to prevent exacerbating soil salinization. Additionally, ammonium chloride should not be applied alongside alkaline fertilizers.
Approximately 90% of ammonium chloride is used in the production of various compound fertilizers. Granular ammonium chloride offers advantages such as mechanized fertilization, blending with other fertilizers to produce BB fertilizer, and prolonged fertilizer effect. Dry granulation is the primary method for producing granular ammonium chloride compound fertilizers.

Benefits of Using Dry Granulation for Ammonium Chloride Fertilizer Making
The Theory of Dry Granulation
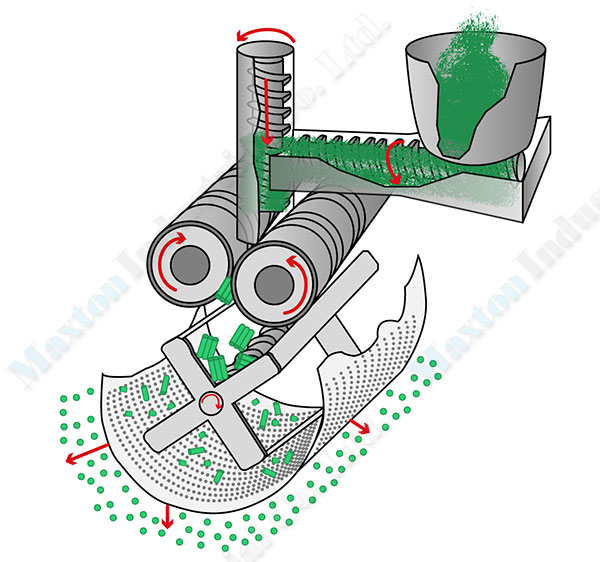
The principle of dry granulation mainly involves the agglomeration of dry material into dense and hard flakes or ribbons under pressure, known as the compaction process. The function of compaction is firstly to expel the air between particles, and secondly, to bring the particles close enough to generate Van der Waals forces, adsorption forces, crystal bridges, and embedded connections. The strength of the granules formed through roller compaction mainly relies on the intermolecular forces. This process is simple, entirely physical, relatively low in investment, only requires electricity as an energy source, has low processing costs, and is particularly suitable for heat-sensitive materials.
Advantages of Granular Ammonium Chloride
- Granular ammonium chloride produced through dry granulation can withstand high pressure, making it easy to transport.
- Granular ammonium chloride can serve as an alternative nitrogen source in mixed fertilizers, addressing the inherent issues with ammonium chloride while improving its storage and transportation properties, thus resolving supply chain inventory shortages.
- High-strength ammonium chloride granules can slow down the dissolution rate of ammonium chloride when applied, providing a slow-release effect and improving nitrogen fertilizer utilization efficiency. Thus, using ammonium chloride granulation technology can reduce processing losses, save production costs, and increase sales profits.
A Dry Granulation Process for Ammonium Chloride Granular Making
Dosing
Raw materials are delivered to the granulation system via a discharge weighing system or a similar setup.
Compaction and Crushing
The raw materials are compacted through a roller compactor. The compacted material flakes then moved to the crushing device under the compactor for crushing.
Screening
The crushed material enters a screening machine via the handling system. Powder and small particles, along with large particles, are returned to the feeding silo for re-compaction, while suitable granules are transported to the product storage hopper via a product conveyor for packaging.
When a rotary drum screener is used for screening, the granules continuously rub against the screen surface, smoothing the edges to a certain degree, resulting in finished granules with relatively smooth surfaces.
Final Product Packaging
The finished granules in the product storage hopper are packaged through an electronic weighing and packaging system.

A typical composition of ammonium chloride granules
| Nitrogen content % | Salinity % | Alkaline substances % | Size (mm) |
| >25.4 | <2.5 | <0.2 | 2-4 |
The process of making granular ammonium chloride is a standard procedure, with typical granule sizes around 2-4mm or according to specific requirements. The system is meticulously designed to avoid future operational issues such as moisture and temperature.





