What is red mud
Around 1888, Austrian chemist Carl Josef Bayer developed a method for producing aluminum oxide from bauxite.
The principle is simple: bauxite is mixed with NaOH solution and heated. The aluminum oxide contained in the bauxite can dissolve into sodium aluminate in the NaOH solution. After filtering to remove the precipitate, aluminum hydroxide seeds (Al(OH)3) are added to the sodium aluminate solution to re-precipitate Al(OH)3. The remaining solution, which mainly contains NaOH, is then reused to process the next batch of bauxite. The obtained Al2O3 can be prepared by calcination. Nowadays, over 90% of the global factories still use the improved Bayer process to produce Al2O3.
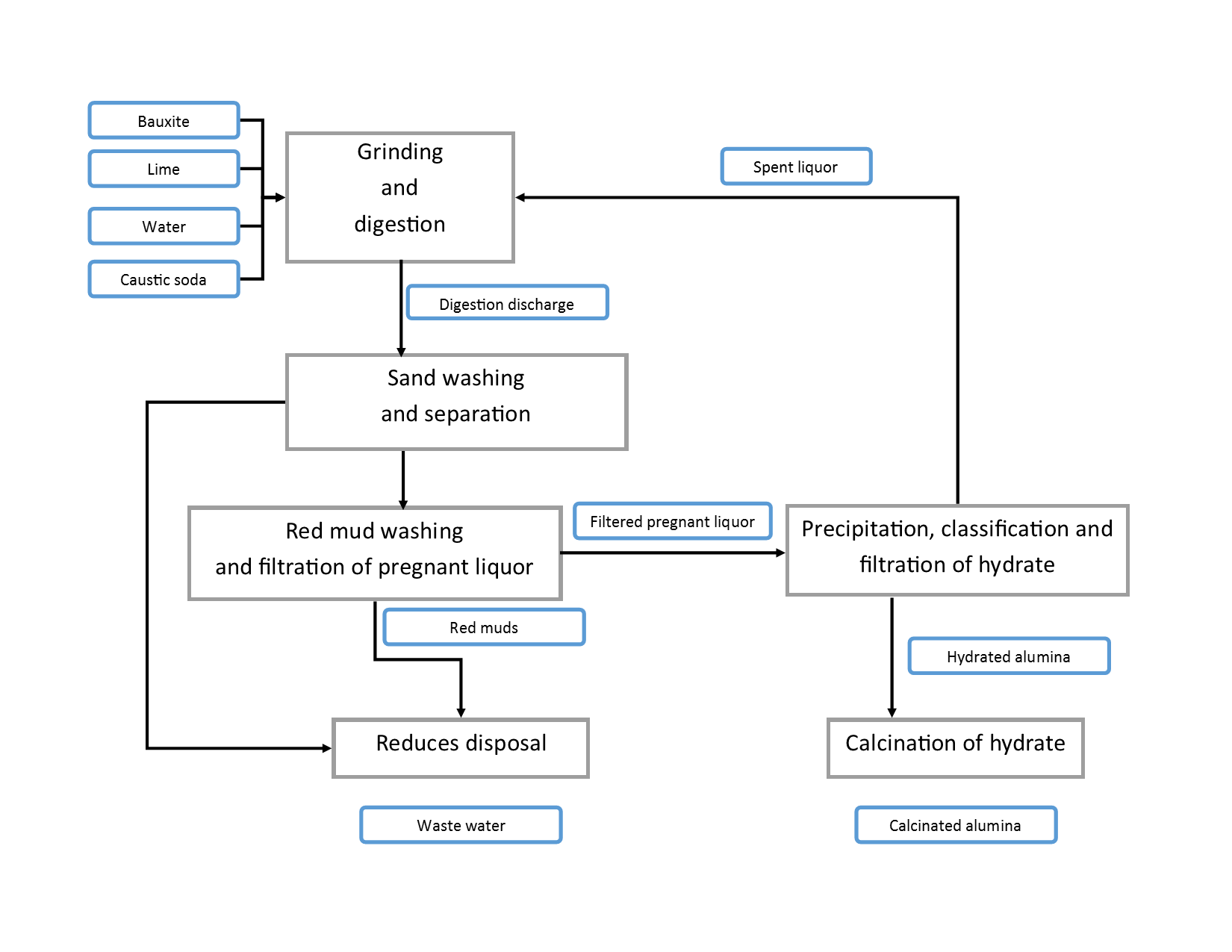
The Bayer process is effective, but it comes with noticeable “side effects” – the precipitates filtered out are difficult to handle. They contain a large amount of iron oxide, as well as silicon oxide, calcium, titanium oxide, and unreacted aluminum oxide, presenting as a reddish-brown color similar to red soil, hence referred to as “red mud.”

Is red mud toxic, the pollution
The red mud and its associated liquid mainly contain pollutants such as alkalis, chlorides, and other substances. The pH of red mud typically ranges from 8.5 to 13. According to statistics, for every 1 ton of alumina produced, approximately 0.7 to 2 tons of red mud will be produced.

The impact of red mud on the surrounding environment of the disposal site mainly manifests in the following aspects:
Water Environment Pollution
Red mud contains pollutants such as alkalis, fluorides, and aluminum ions, causing water bodies’ pH levels to rise and exceeding permissible limits for suspended solids and harmful impurities. These pollutants infiltrate groundwater through various pathways, entering the human body through the food chain or prolonged consumption of contaminated groundwater, affecting human health. The dumping of solid red mud into rivers, lakes, or oceans directly pollutes water bodies, endangering aquatic life and hindering water resource utilization. Additionally, runoff, leaching, decomposition of red mud, and the conversion and migration of harmful substances severely pollute surrounding rivers and groundwater. Dumping solid red mud into water bodies also reduces their effective surface area, leading to decreased drainage and irrigation capabilities.

Soil Environment Pollution
Large quantities of land and farmland are occupied by red mud disposal, leading to soil marshification and salinization. The presence of significant amounts of pollutants alters soil properties and structure, causing widespread soil marshification and salinization. This adversely affects soil microbial activity and inhibits plant growth and development. Pollutants in red mud can accumulate in plant tissues and enter the human body through the food chain, posing health risks. Harmful components like alkalis in red mud can inhibit plant growth and development, leading to surface soil erosion in areas lacking vegetation.

Atmospheric Pollution
The drying of surface red mud in disposal sites generates airborne dust, contributing to atmospheric pollution. Fine particles containing alkalis and other harmful components become airborne in the wind, increasing airborne dust levels and inhibiting plant growth. Studies indicate that during wind speeds of four or higher, particles with diameters of less than1.5cm become airborne, reaching heights of 20-50m.

Red mud composition
Due to differences in the source of bauxite, production processes, and equipment, red mud compositions vary, comprising both chemical and mineral components.
Chemical components mainly include Al2O3, Fe2O3, SiO2, CaO, Na2O, TiO2, and trace amounts of rare metals, rare earth elements, and radioactive elements.
Additionally, in the red mud obtained from several production processes, the content of Na2O is relatively high, which is the primary reason for the alkalinity of red mud.
The mineral composition of red mud is quite complex, typically containing minerals such as:
Hematite (Fe2O3) and goethite (α-FeO(OH))
Quartz (SiO2·nH2O)
Sodium feldspar (Na2O·Al2O3·1.68SiO2·1.73H2O)
Cancrinite (3NaAlSiO4NaOH)
Hematite
Rutile (TiO2)
Boehmite (AlO(OH))
Gibbsite (Al(OH)3)
Calcite (CaCO3)
Hydrogrossular garnet (3CaO·Al2O3·xSiO2–(6−2x)H2O)
The content of each component varies significantly depending on the production process, including the Bayer process, sintering process, and combined process. For example, compared to the sintering process, red mud produced by the Bayer process typically has lower silicon and calcium content but higher levels of iron (Fe), aluminum (Al), and sodium (Na).

Main components of red mud from different origins
| Origin | Fe2O3 | Al2O3 | SiO2 | TiO2 | Na2O | CaO | MgO | Na2O |
| Australia | 60 | 15 | 5 | 5 | 16 | – | – | – |
| New Zealand | 45.58 | 15.65 | 6.96 | 7.07 | 3.26 | 14.84 | – | 0.07 |
| Turkey | 30.45 | 17.91 | 9.58 | 8.61 | 12.06 | 7.77 | 0.86 | 0.03 |
| Jamaica | 35.73 | 23.29 | 12.08 | 4.08 | 7.4 | 2.81 | 0.76 | 0.28 |
| Spain | 45.3 | 18.8 | 4.3 | 6.4 | 1.5 | 3.1 | – | – |
| China | 47.5 | 11.93 | 1.87 | 5.06 | 1.4 | 0.68 | 0.11 | 0.045 |
Main components of red mud from different processes
| Method | Al2O3 | Fe2O3 | SiO2 | CaO | Na2O | K2O | MgO | TiO2 | ignition loss |
| Bayer | 10-20 | 30-60 | 3-20 | 2-8 | 2-10 | – | – | 0.1-10 | 10-15 |
| Sintering | 5-7 | 7-10 | 20-23 | 46-49 | 2-2.5 | 0.2-0.4 | 1.2-1.6 | 2.5-3 | 6-10 |
| Bayer-sintering combined | 5.4-7.5 | 6.1-7.5 | 20-20.5 | 43.7-46.8 | 2.8-3 | 0.5-0.7 | – | 6.1-7.7 | – |
5 ways to recycle red mud
Based on the characteristics of red mud, its utilization can be primarily categorized into four aspects:
Recovery and Utilization of Valuable Metals: Red mud contains valuable metals such as iron, aluminum, titanium, and rare earth elements, which can be extracted for their economic value.
Preparation of Building Materials: With its high content of calcium, silicon, and aluminum in the form of silicate minerals, red mud can serve as raw material for construction materials.
Production of Environmental Materials: Due to its alkalinity and adsorption properties, red mud can be utilized in the production of environmentally friendly materials.
Manufacturing Flame Retardant Materials: Red mud, with its high surface area and content of elements like silicon, aluminum, iron, and titanium, can be used to produce flame retardant and firefighting materials.
Making cooling agents and slagging agents: The iron and other related elements extracted from red mud can be further processed and used as coolant and slag conditioner in steel mills.
Recovery and Utilization of Valuable Metals
Red mud is rich in Al2O3 and Fe2O3 content. Although the elements present in red mud vary depending on the source, almost all red mud contains various types and quantities of metal elements, indicating significant potential for the recovery and utilization of valuable metals.
Recovery of Iron and Aluminum
The iron content in red mud ranges from 5% to 50% and is primarily found in minerals such as hematite, goethite, and ferrihydrite. Methods for iron recovery from red mud include magnetic separation and wet metallurgy.
The aluminum content in red mud ranges from 4% to 30% and is primarily present in minerals such as gibbsite and bayerite. Methods for aluminum recovery from red mud include the “calcification-carbonization” process and wet metallurgy.
Recovery of Rare Metals
Rare earth elements refer to a group of 17 special elements widely used in fields such as luminescent materials, aerospace, batteries, and defense industries. In addition to major components like Al2O3, Fe2O3, SiO2, TiO2, and CaO, red mud also contains rare metals such as V, Sc, Ni, and Ga. Studies have shown that rare earth elements in red mud primarily exist in minerals such as calcium-aluminum garnet, hematite, goethite, ferrihydrite, gibbsite, and bayerite. In the Bayer process, approximately 98% of rare earth elements transfer to the red mud. With advancements in science and technology, the recovery of rare earth elements from red mud holds significant research value.
The main method for recovering rare earth elements is through leaching and extraction. During the recovery process, steps such as separating impurities and extracting the leached phase are required to enrich and recover the rare earth elements. Therefore, the focus is primarily on optimizing the leaching and extraction conditions for efficient recovery.
Preparation of Building Materials
Currently, the most significant utilization of red mud is in the preparation of building materials. Due to its composition containing SiO2, Al2O3, CaO, etc., red mud can be used to produce cement, lightweight aggregates, bricks by the red mud brick making machine, or as subbase material for road construction. The market for building materials is extensive. By using red mud for building materials while ensuring the safety of its reserves, not only can a large amount of red mud be consumed, but also the usage of cement and other building materials can be reduced, leading to a decrease in carbon emissions.

Furthermore, utilizing the components such as SiO2, Al2O3, CaO, etc., in red mud for the preparation of ceramic materials and microcrystalline glass is also a worthwhile research direction.
Preparation of Environmental-friendly Materials
Due to its high pH value, fine particle size, high porosity, and rich iron oxide content, red mud exhibits excellent adsorption properties. Red mud can be used not only for metal extraction and the preparation of building materials but also for producing high-performance inorganic environmental-friendly materials for wastewater treatment, air purification, and as a soil amendment for soil modification.
When using red mud for wastewater treatment and soil remediation, it’s essential to note that red mud itself contains some heavy metal ions, which may leach out during usage. Therefore, pretreatment of red mud is necessary before utilization, and further research is required to enhance the stability of environmental-friendly materials based on red mud.

Manufacturing Flame Retardant Materials
Aluminum-based, silicon-based, and other flame retardants are favored in the field of firefighting due to their non-toxic, environmentally friendly nature, good stability, and ability to reduce smoke emission during plastic combustion. Red mud contains high levels of substances such as SiO2, Al2O3, Fe2O3, TiO2, which exhibit excellent flame retardant properties. Therefore, red mud can be utilized as a flame retardant and extinguishing agent in the firefighting domain.
Agglomeration
The adaptability of electric arc furnace smelting to raw materials is quite broad. However, due to the fine particles of Bayer process red mud, excessive direct entry of powder into the furnace leads to poor permeability of the charge, making it difficult for smelting gases to escape. This worsens furnace conditions, impacting various technical and economic indicators. Therefore, it is essential to preprocess Bayer process red mud by pelletization. Cold agglomeration of Bayer process red mud produced by disc pelletilizer and roll briquetting can be used to treat the red mud. The product can replace some ores as cooling agents and slag conditioners in converter steelmaking.

In addition to conventional cold agglomeration, Geopolymer-based solidification provides another promising route for treating Bayer process red mud. Unlike purely mechanical pelletization, this method uses alkali-activated aluminosilicate binders to chemically immobilize fine red mud particles. When combined with Maxton’s advanced briquetting technology, the Geopolymer reaction forms a dense three-dimensional network, resulting in briquettes with high compressive strength, long-term water stability, and excellent resistance to elevated temperatures.
With Maxton as a provider of integrated briquetting solutions, such chemically stabilized briquettes are not only suitable for metallurgical use as slag conditioners but can also serve as sustainable construction aggregates, replacing natural stone and refractory products.
Compared to traditional cold agglomeration, Geopolymer-based agglomeration addresses two critical issues simultaneously:
- Safe immobilization of hazardous elements (such as Na₂O and heavy metals) contained in red mud, preventing leaching and secondary pollution.
- Value-added utilization of waste, producing construction-grade materials with durability comparable to natural aggregates.
Through the integration of Maxton’s briquetting line and Geopolymer solidification technology, this advanced approach to red mud agglomeration provides a holistic solution — not only a metallurgical raw material substitute, but also a pathway to circular economy applications in the building materials sector.





