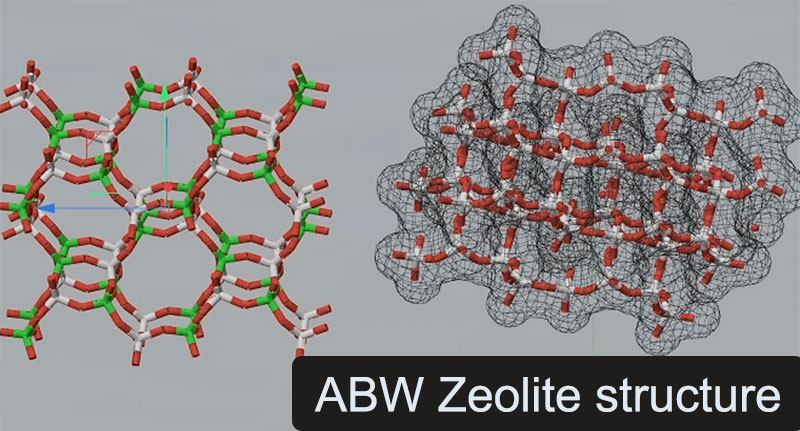
Discovered of “us”
It was first discovered by the Swedish mineralogist Axel Fredrik Cronstedt in 1756 in the Svappavari copper mine in Lappmak, Sweden, that a unique shelf-like aluminum silicate mineral crystal had a boiling phenomenon when heated during blowpipe analysis, so these minerals were given Named “zeolite”, which means “boiling (zeo) stone (lithos)” in Greek, this is our family – the Zeolite tribe. Later, our tribe became famous all over the world with the name “Zeolite”.
Our body composition can generally be expressed like below
AmXpO2p·nH2O
Various zeolite members have different shapes, some are fibrous, columnar, plate-like, and some are granular;
The skin is usually white or colorless, and sometimes it is dyed into other colors if it contains impurities;
There is generally a set of complete cleavages;
We have many family members, such as:
- Mordenite
- Analcite
- Heulandite
- Chabazite
- Clinoptilolite
- Ferrierite
- Erionite
- Natrolite
- Stilbite
- and other zeolite minerals, etc.
Not only do they have complex body structures, but they are also naturally beautiful and have incredible looks.
Mordenite
Origin of the name: It was first discovered and named by Henry How in 1864 in a small community in Nova Scotia, Canada.
Chemical composition: In most cases, there are more alkali metal elements than Ca, and more Na than K.
Crystal characteristics: Orthrhombic crystal system, often fibrous crystal form. The high ratio of silicon atoms to aluminum atoms in the molecular structure of NSi/NAl mordenite makes it more resistant to acid attack than most zeolites.
Physical properties: Common colors are white, light yellow, pink or colorless. Its Mohs hardness is 5 with density of 2.1g/cc.





Analcite
Analcite is often classified as a zeolite mineral, but is structurally and chemically more similar to the feldspar group.
Chemical composition: The chemical formula is Na2[AlSi2O6]2·2H2O, sometimes containing a small amount of K, Ca or a small amount of Mg.
Crystal structure: Equiaxed crystal system, shelf-like structure
Main Origin: Analcite is commonly found in basalt and other alkaline igneous rocks. Major production locations include Croft Quarry in Leicestershire, England; Independence Island in eastern Sicily and near Trentino in northern Italy; Victoria; Kerguelen Island in the Indian Ocean; and Lake Superior in Michigan The Copperfields, Bergen Mountain in New Jersey and Lake Serres in California, USA; Nova Scotia, Quebec, Canada and Cape Blomidon in Cape Saint-Hilaire.







Natrolite
Chemical composition: The chemical formula is Na2[Al2Si3O10]·2H2O, which belongs to the framework silicate mineral of the zeolite family. It is a kind of hydrated sodium silicate and aluminum silicate.
Origin of the name: It was originally named natrolite by Martin Heinrich Klaproth in 1803. Derived from the Greek word for soda, highlighting its high sodium content. Needlestone or needleite is another informal name that emphasizes its common needle-like morphology, which usually develops into very fine, aggregated clusters.
Physical Properties: Natrolite is usually white, colorless or pink, but sometimes reddish or light yellow. It has a glassy luster.









Chabazite
Origin of the name: It was named chabasie by Bosc d’Antic in 1792, and later changed to chabazite.
Crystal Characteristics: Trigonal crystal system, crystals are usually twins, and contact twins and penetration twins can be observed.
Physical Properties: They can be colorless, white, orange, brown, pink, green or yellow. The hardness range is 3 to 5, the specific gravity is 2.0 to 2.2, and the glass luster is.
Main Origin: Chabazite is most commonly found in the voids in basalt. Chabazite is often produced in India, Iceland, the Faroe Islands, the Giant Causeway in Northern Ireland, Bohemia, Italy, Germany and other places.






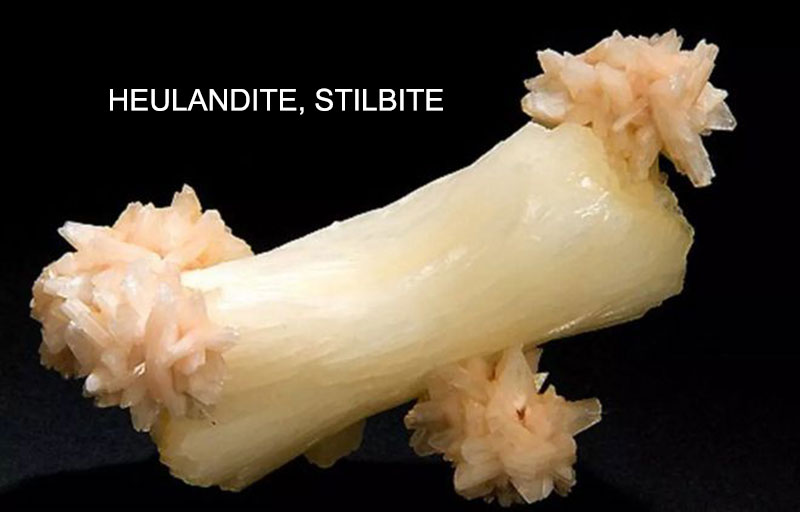
Stilbite
Origin of the name: Sometimes, heulandite and stilbite are considered to be the same mineral. After it was discovered in 1818 that it was two separate minerals, the name “stilbite” was used, from the Greek stilbein, meaning “to shine”, because stilbite has a pearlescent luster and looks dazzling.
Crystal Characteristics: Crystals usually develop into thin flat plates, and the aggregates can be bundles or bundles, or fibers and spheres.
Physical Properties: The color is usually colorless or white, but also yellow, brown, pink, salmon, orange, red, green, blue or black. Usually it has a glass luster, and the cleavage surface shows a pearly luster. The stripes are white and the crystals are transparent to translucent. The hardness is 3 to 4 and the specific gravity is 2.12 to 2.22. {010}Complete cleavage. Crisp, with shell-like or uneven cracks.
Main Origin: Stilbite is a low-temperature secondary hydrothermal mineral. It is commonly found in amygdala cavities in basaltic volcanic rocks, andesites, gneisses, and hydrothermal veins. The main producing areas are Iceland, the Faroe Islands, the Isle of Skye, the Bay of Fundy, Nova Scotia (provincial minerals), northern New Jersey and North Carolina. The volcanic rocks contain abundant Stilbite.










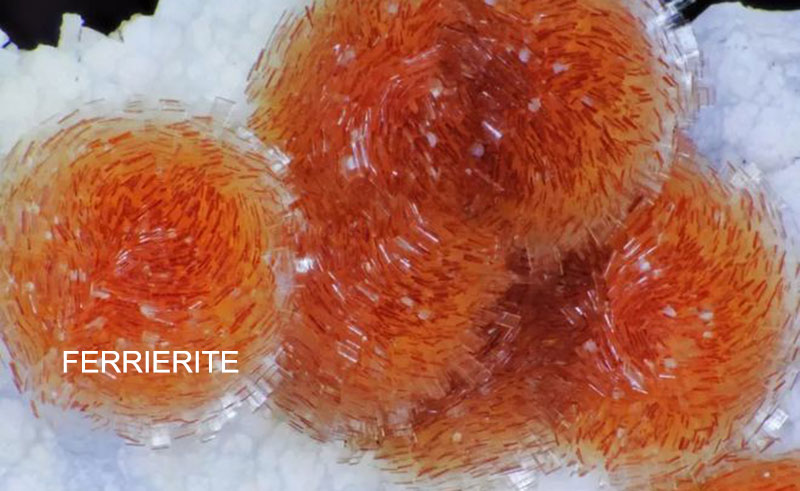
Ferrierite
Origin of the name: Ferrierite is named after Canadian geologist and mining engineer Walter Frederick Ferrier (1865-1950).
Crystal Characteristics: Orthrhombic crystal system. The crystals are often long plate-shaped and needle-shaped.
Physical Properties: The relative density is 2.14~2.21, the refractive index is 1.479, and the hardness is 3~3.25. The molar ratio of silicon to aluminum in natural ferrierite is usually around 12.








Heulandite
Crystal Characteristics: The crystal is monoclinic. They may have the characteristic coffin shape, but may also form simple rhombohedral prisms, sometimes with a layer of fine wedge-shaped crystals.
Physical Properties: Minerals are usually colorless or white, may be orange, brown, yellow, brick red or green, with a glassy luster, it ranges from transparent to translucent.









Other







The body structure of our family is very different from other shelf-like silicate tribes. There are wide cavities and channels in our body structure, which can absorb some metal cations and water molecules to form zeolite water. Zeolite water participates in the synthesis of our body structure. However, when the temperature rises to 80°C to 400°C, the zeolite water will begin to escape and completely dissipate, and certain physical properties of our body will change accordingly, such as transparency, refractive index, relative density and other physical properties. Decreases with increase in water loss. After losing the zeolite water, it can still absorb water again, thereby restoring the previous physical quality. This special body structure has made our family highly valued by humans. We have extensive and unique applications in industry, agriculture, national defense and cutting-edge science and technology.
Applications
Ion exchange material
Cations located in the wide cavities and channels of the zeolite family can be replaced by other cations without destroying the crystal structure. For example, the 2Na+ provided by zeolite is used to exchange Ca2+ to soften the hard water originally containing high Ca2+. It can also desalinate seawater or extract K from seawater. It can be used for wastewater treatment to remove harmful ions such as radioactive elements and heavy metal elements in wastewater.
Molecular sieve
The ions located in the wide cavities and channels of the zeolite family can reabsorb ions after they are lost, and ions with diameters larger than the channels will be rejected, thereby achieving selective absorption and screening molecules. Molecular sieves using zeolite can separate mixed gases and liquids, remove waste gas, process natural gas, etc., and can also be used for soil improvement, that is, nutrients are adsorbed in the zeolite crystal lattice and are not easily lost and are only slowly absorbed by plants.
Cement and building materials industry
In addition, we can also be used in the cement and building materials industries to make strong and lightweight products. Due to the widespread use of zeolite minerals, naturally produced zeolites can no longer meet human needs. Volcanic rocks (perlite, rhyolite, etc.) and clay rocks are currently used to synthesize zeolites. Minerals such as kaolinite, pyrophyllite, etc. can also be used to synthesize zeolites. This has formed a very experienced production line.
Granular making
Zeolite is widely used as a fertilizer and soil amendment for it’s ability to increase water retention in the soil. Clinoptilolite increases soil porosity for aeration, helps prevent soil compaction, and is a carrier of essential nutrients and water. Able to hold up to 55% of its own weight in water, zeolite is able to conserve water from irrigation and rainfall so that nutrients and water are held in the root zone and readily available to plants for fast growth.
Bentonite and zeolite compound granular is also made via a dry granulation or pelletizing process to make qualified cat litter for our pets.