How is the element silicon formed?
During the cosmic Big Bang, the abundance of silicon was only surpassed by hydrogen, helium, oxygen, neon, nitrogen, and carbon. Silicon is considered a cosmic product formed through the absorption of alpha particles at temperatures around 10^9 Kelvin. It is composed of atomic nuclei of carbon-12, oxygen-16, and neon-20. The particle energy required for the formation of silicon nuclei is approximately 8.4 million electron volts (MeV) per nucleon (proton or neutron). In comparison, iron nuclei have a maximum energy of around 8.7 million electron volts and nearly twice the atomic mass of silicon, indicating the relative stability of silicon nuclei.
Why is silicon so abundant in the Earth’s crust
Silicon is an element widely distributed in our Earth’s crust, accounting for 27.7% of the crust’s mass, second only to oxygen and ranking as the second most abundant element in our crust. Si is a fusible component, and during the melting process, Si tends to enter the melt. Therefore, in the partial melting processes of the crust or mantle, Si easily enriches in the melt, ultimately leading to an increased abundance of silicon in the Earth’s crust.
How was silicon discovered
Due to silicon’s strong chemical affinity for oxygen, it wasn’t until 1823 that Swedish chemist Jöns Jacob Berzelius first isolated silicon. He achieved this by heating a mixture of metallic potassium and potassium fluorosilicate in a crucible, resulting in the formation of amorphous silicon, which he described as a new element.
Natural silicon
In nature, it’s rare to find elemental silicon on its own; it always prefers to be with its companions. One of its best friends is oxygen. Silicon often combines with oxygen to form silicon dioxide (SiO2), and many beautiful and interesting things happen as a result.

Nature presents a diverse array of silicon dioxide compositions, showcasing itself in various forms. Colorful agate, sandy shores, beautiful crystals, sparkling quartz, unique chalcedony, and dense, hard flint – all share the common essence of being composed of silicon dioxide. The variations of mineral crystals naturally formed from silicon dioxide have hundreds of names. To geologists, silicon dioxide might be quartz, flint, grit, sand, or coesite, among others. For those who appreciate jewelry, it can manifest as amethyst, quartz, agate, gray chalcedony, or chrysocolla, to name a few. 






Silicon in human history
There are many vivid and interesting stories related to silicon dioxide. The Egyptians used quartz to make beads and vases. In ancient China, delicate glassware was crafted from it. Ground crystal has been utilized as an art form, seen in items such as crystal flutes displayed in the Vienna Art Museum or Russian-style transparent teapots preserved in the Moscow Armory Museum.

Flint, dense and hard, was one of the earliest minerals used by ancient humans when they began crafting tools. Our ancestors intentionally struck it to create knives, awls, and axes, essential for obtaining food, felling trees, removing branches, and processing animal skins into clothing, tents, containers, and more. Striking it with iron tools produces sparks, making it valuable for starting fires in the wild. Of course, other hard rocks capable of producing sharp materials were also utilized in tool-making.

In the 20th century, quartz triggered a revolution in timekeeping. Swiss watchmaking had dominated for nearly a century until 1969 when Japan introduced the first quartz watch – the Seiko 35SQ Astron. It utilized a battery-powered quartz crystal resonator, shaking the stronghold of Swiss watches. However, in 1983, the Swiss regained market share globally by establishing their own quartz watch brand – Swatch.
Besides silicon dioxide, silicon can also form silicates by combining with other metals.





It is also one of the most useful elements for humans. Have you noticed it in your daily life?
Silicon dioxide and silicates are used in civil engineering projects for manufacturing concrete and cement, as well as in the production of enamel, pottery, porcelain, and other ceramic materials.
Silicon dioxide can be used in the manufacturing of refractory materials for high-temperature applications, such as quartz crucibles. It can be heated to soften and formed into glassware. Silicon dioxide is also utilized as an abrasive, for example, in the form of quartz sand. Additionally, it is used in the production of fibers made from glass or plastic and for thermal insulation in the form of glass wool.
Sodium silicate, commonly known as water glass, is used in various applications. It is employed in soap making, wood preservation, and egg preservation. Additionally, it serves as a binding agent in the agglomeration field for briquetting, compacting, etc.
Carbide silicon is an important abrasive material and is also used in laser area.
Mostly used in the manufacturing of alloys, including aluminum-silicon and ferrosilicon. They are employed in the production of generator and transformer plates, engine cylinder blocks, cylinder heads, machine tools, and for deoxidizing in steel making.
![]()
Silicon is also used in the manufacturing of silicone, commonly known as silicone oil or dimethyl silicone oil, which is a silicone-oxygen polymer with methyl groups. Silicone oil serves as a lubricant and is added to some cosmetics and hair conditioners. Silicone rubber is utilized as waterproof sealant in bathrooms, windows, pipes, and around roofing.
![]()
The element silicon is the foundational material for the semiconductor industry. Many places worldwide are named “Silicon Valley.” Silicon, as a semiconductor, is extensively used in the solid-state devices of the computer and microelectronics industry. Silicon is selectively doped with small amounts of boron, gallium, phosphorus, or arsenic to control its electrical properties.
Silicon in the bodies of animals and plants
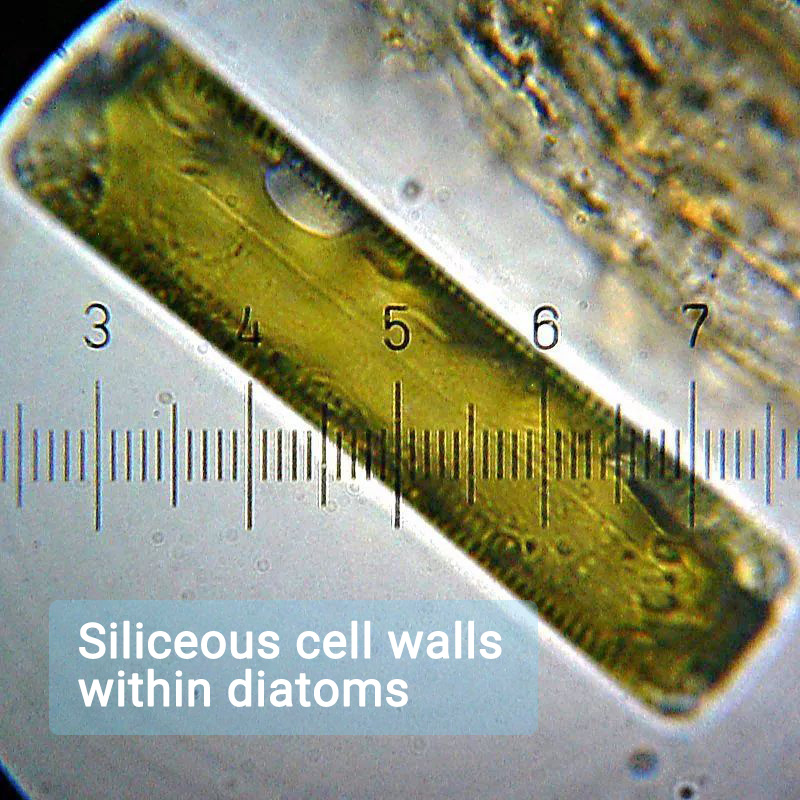
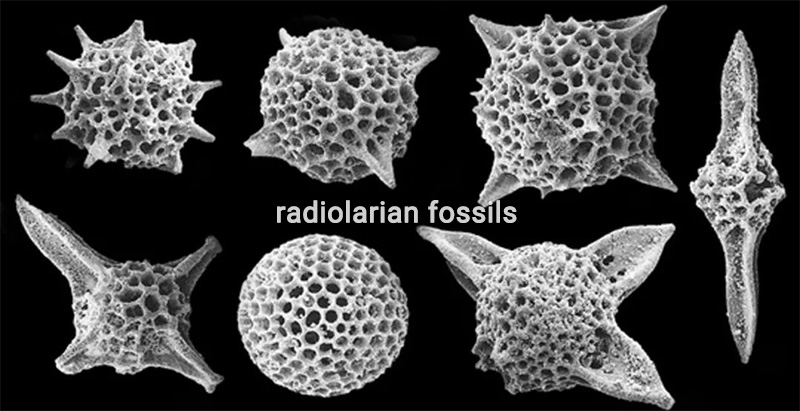
Diatoms, radiolarians, and siliceous sponges use biogenic silica as their skeletons or shells. Radiolarians, for instance, have a unique shell composed of fine needle-like silica. Some plants require silicon for growth, such as tall-stemmed plants that need silicon to maintain rigidity. Silicon has been shown to enhance the cell wall strength and structural integrity of certain plants. Diatoms are algae that undergo photosynthesis, and what sets them apart is that their cell walls are made of silica.