Table of Contents
ToggleWhat is a “mineral”?
A mineral is a naturally occurring substance found in the Earth’s crust,
formed through geological processes,
with a defined chemical composition and physical properties,
existing as elements or compounds.

Chapter One: Super Element Factory
Did you know?
Every piece of gold on Earth
originated from the stars in the universe.
Today, let’s talk about their origins.
After reading this article,
maybe your dream of having a mine at home might come true.
First, let’s review a school concept.
We know
this world
is composed of various chemical elements.
First is hydrogen,
then helium.
They look something like this:

Hydrogen floats everywhere in the universe.
In terms of abundance,
it makes up about 90%.
Where does all this hydrogen come from?
According to the Big Bang theory,
they all originated from a massive explosion 13.82 billion years ago.
A dense, hot singularity
suddenly exploded for unknown reasons.
Although the explosion was powerful,
it could only produce hydrogen and helium.

To get the other elements in the periodic table,
we rely on the element factories of the universe:
stars.
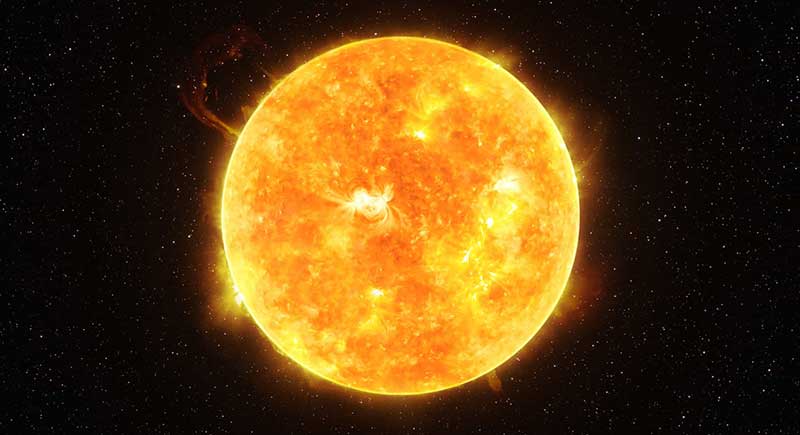
Under the influence of gravity,
hydrogen gathers together
and is heated to extremely high temperatures.
Then,
two hydrogen atoms fuse
in a nuclear fusion reaction,
producing a new element:
helium.
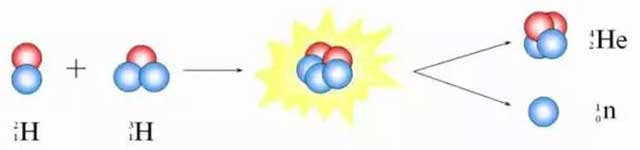

This process releases an enormous amount of energy,
which is also the principle behind the hydrogen bomb.
Countless “hydrogen bombs” are exploding every moment.
As the temperature inside the star rises
to over hundreds of millions of degrees Celsius,
new fusion reactions occur.
Helium fusion begins.
The newly generated elements become more complex.
Sometimes, the star becomes a red giant.

This way, all elements from helium to iron (atomic number 26) are formed.
Some massive stars,
in the late stages of their life cycles,
start fusing iron,
which leads to a colossal explosion
known as a supernova.

This explosion forms elements from cobalt (27) to uranium (92),
including our favorite: gold (79).
But the price is high—
To produce the gold on Earth,
a star at least eight times the size of the sun must explode!
Then,
starting from element 93 (neptunium),
the universe can’t create them naturally anymore—
they are all man-made.
Isn’t humanity amazing?

After the supernova explosion,
various elements are scattered throughout space.
Of course,
hydrogen still dominates.
Under gravitational forces,
these substances gather once again
to form new stars and planets.
Our solar system was formed this way.
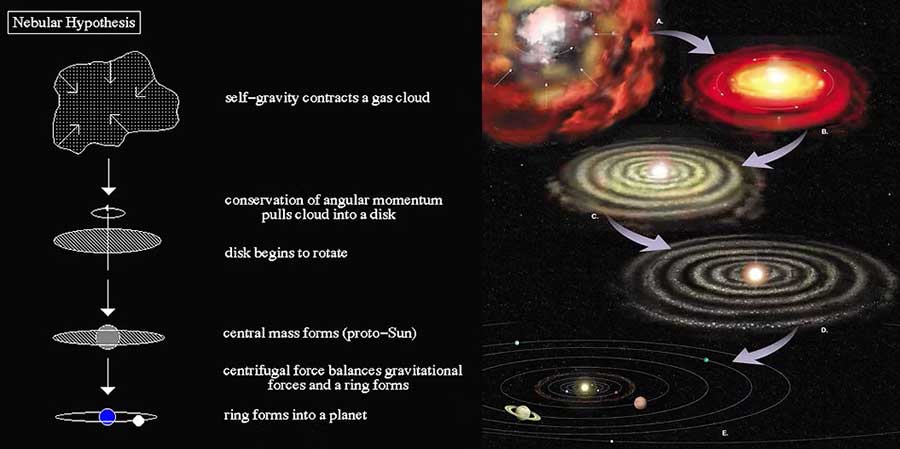
The sun is about 5 billion years old,
relatively young compared to the universe.
The materials in our solar system
come from the remains of countless past supernovae.
Poetically speaking…
The sun has already gone through several lifetimes.
Chapter Two: Planetary Miners
About 4.6 billion years ago, the Earth was formed.
Luckily, because of its relatively close distance to the Sun,
the Earth contains relatively heavier elements due to gravitational sorting.
Farther planets, on the other hand, are composed mostly of lighter elements.
For example, Jupiter is mainly made of hydrogen and helium.
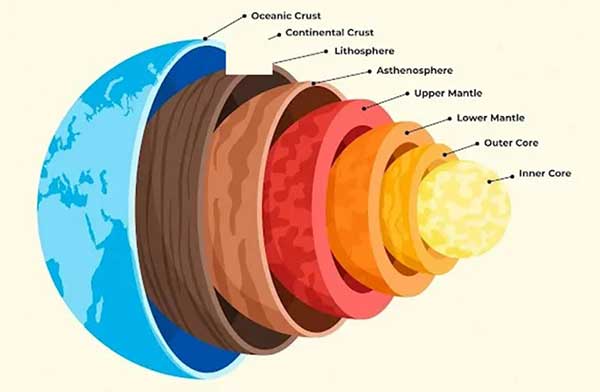
The internal structure of Earth looks like this:
At the center is the inner core composed of iron and nickel,
similar to the composition of many iron meteorites in the solar system.
In the Earth’s crust, the relative abundance of elements is approximately:
-
Oxygen 48.60%
-
Silicon 26.30%
-
Aluminum 7.73%
-
Iron 4.75%
-
Calcium 3.45%
-
Sodium 2.74%
-
Potassium 2.47%
-
Magnesium 2.00%
-
Hydrogen 0.76%
-
Others 1.20%
The gold everyone loves
is hidden within this remaining 1.2%—
and in extremely small amounts.

The Earth is so big,
but gold is so scarce.
If it were evenly distributed throughout the Earth,
how would we even find it?
Is there a way
to make gold gather together on its own?
The answer is yes—
through volcanoes.
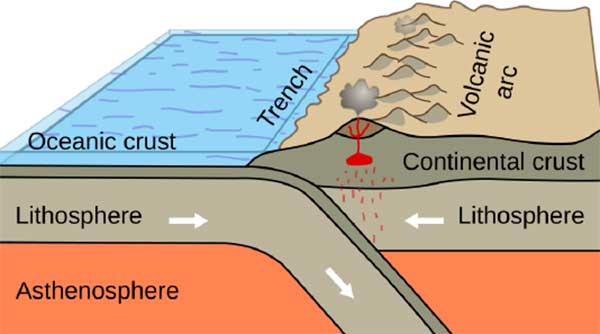
As we know, tectonic plates move.
The hard lithosphere floats on the softer asthenosphere,
moving slowly.
Sometimes, oceanic plates subduct under continental plates.
In this process,
a large amount of water is brought underground along with the subduction.
Under high temperature and pressure,
this water is heated to over 500°C.
This extremely hot water
can dissolve many metal elements,
including gold, silver, copper, and iron—
minerals we’re all familiar with.
This high-temperature fluid is called
hydrothermal solution.
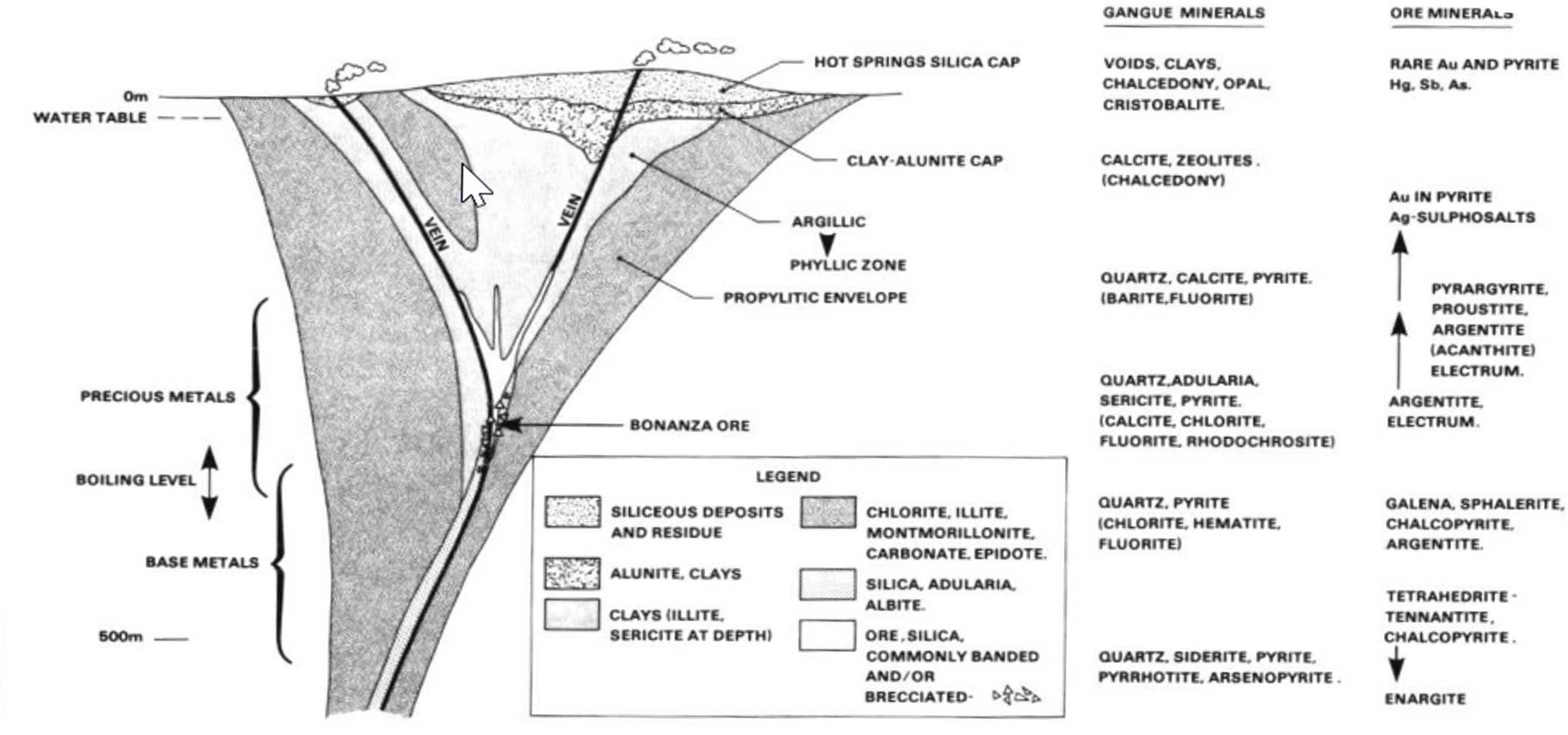
When hydrothermal fluids rise through volcanic conduits,
they gradually cool down.
As they cool,
different minerals precipitate out sequentially.
When cooled to around 200°C–300°C,
gold deposits form.

Chapter Three: Humanity Appears
After a long evolutionary process,
life evolved from simple to complex,
and eventually, humans appeared on Earth.
The history of human civilization
is essentially a history of using minerals.

This is a beautifully crafted stone tool,
made from a material commonly found across the planet: quartz.
Yes, it’s everywhere.
Our ancestors could easily find this material.
It’s hard and fractures sharply—
perfect for making weapons.
But it had one weakness—
it was too brittle.

Also, using stone tools wasn’t very advanced.
At least four or five animal species can also use tools.
Soon, humans discovered better materials.

Then came the Bronze Age,
and later the Iron Age.
As human civilization progressed,
more and more minerals were discovered and utilized.
And very naturally,
as demand increased,
mining and the mining industry emerged.
Organized, large-scale extraction of required minerals began.

Chapter Four: Minerals and Civilization

In North America,
someone discovered a massive metal lump in the forest.
It was a chunk of pure copper.
During the last Ice Age,
a glacier had transported it from a distant location.
After the ice melted,
it remained behind.
Later, people realized
it was a massive piece of copper.
Copper is one of the first metals used by humans.
It is widely distributed
and makes up about 0.01% of the Earth’s crust.
Its usage by humans
led to the Bronze Age.
Later,
its ductility, conductivity, and thermal properties
made it essential in the electrical age.

Copper coins were once among the most important currencies in history,
and used in huge quantities.
So where did all this copper come from?

Both in ancient and modern times,
major copper deposits have been concentrated in certain regions of the world.
These can be seen within the area shown on the map below:
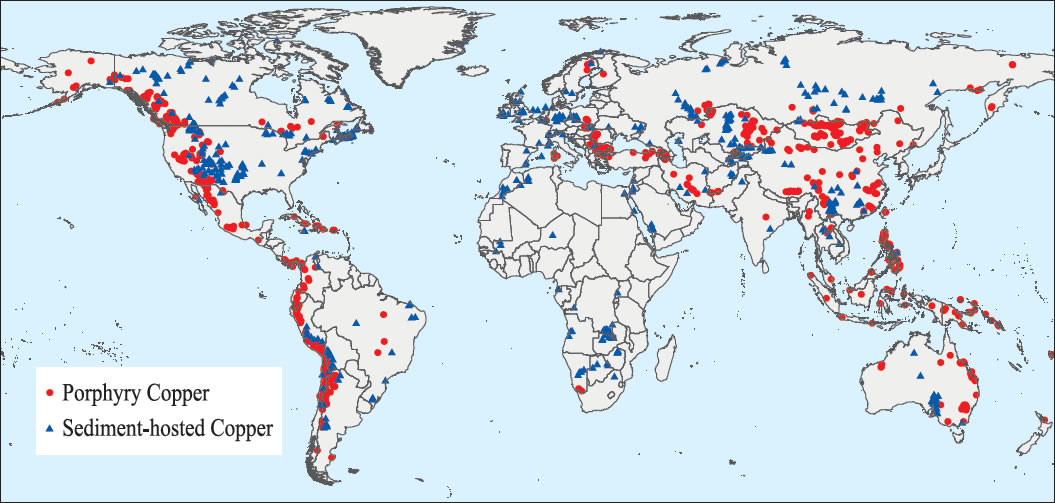 To find copper ore and its companion mineral malachite,
To find copper ore and its companion mineral malachite,
researchers have examined deposits rich in these materials.
In some mines, ore is extracted from deep underground
and brought to the surface by winches and mining carts through inclined shafts.

Among the ore piles,
samples of copper-bearing minerals are often visible.
One of the most common is chalcopyrite,
containing iron, sulfur, and copper.
Unlike other copper ores,
chalcopyrite can display beautiful rainbow colors
caused by thin oxidation films—
an iridescent effect.

After a complex smelting process,
copper is finally produced.
As human civilization continues to advance,
aluminum, a strong yet lightweight metal, emerged.
Its usage now exceeds that of copper.
Airplanes, rockets, and mobile phones
all rely heavily on it.
But it has only been used for a little over 100 years.

Aluminum mainly comes from a mineral called gibbsite.
Its crystals are tiny
and often form spherical clusters.
Typically white,
sometimes blue,
with beautiful glassy luster.

This sample of gibbsite comes from China.
Imagine crushing and melting it
to make your phone case—
wouldn’t you hesitate?
In fact,
industrial-grade bauxite is mostly ugly rock,
but these fine crystals
are carefully excavated and sold as specimens.

As human civilization continued to advance
the use of minerals eventually reached the end of what nature could provide
The 92nd element
Uranium
![]()
Its history of use is even shorter than that of aluminum
In 1945, a single explosion
marked the beginning of the nuclear age
Today, these uranium ores
provide a continuous source of electricity
In certain regions, this important ore is produced and it looks
like this

This beautiful mineral is called autunite
Under natural light, it shows a striking yellow-green color
When exposed to ultraviolet light
It emits an eerie glow
A bright green, like that of a light bulb
This phenomenon is called fluorescence

Today, such mineral crystals have become highly sought-after collectibles
A single specimen can often sell for a very high price
It is also one of the very few minerals that possess radioactivity
Of course, for those who may feel uneasy
and lack sufficient knowledge about radioactivity
It is recommended to seek proper advice
The vast majority of minerals are perfectly safe

Apart from industrial uses
Some minerals serve the purpose of bringing joy, even brightening a person’s day
Besides the well-known diamond
There are many other mineral gemstones
For example, garnet, which is inexpensive yet very beautiful

It truly resembles pomegranate seeds
Its name, garnet, comes without surprise
The garnet family includes many different varieties
Many of them are used in making jewelry
For example, this variety
Spessartine, often called “Fanta garnet” for its vivid orange color

There are also some minerals
That have little industrial value
And cannot be made into jewelry because of their low hardness
Yet
Many people still pay high prices to collect them
Why is that?
Like this enthusiast who went through great effort
To dig up this kind of stone in the desert


It has a very descriptive name
Desert Rose
Its composition is gypsum
The same gypsum used for plaster casts and wall coatings
In desert and arid environments
These flower-like crystals often form
When travelers first discovered them in the desert
They naturally named them Desert Roses

Today, people collect them
Because of their beauty
And because their origin holds fascinating scientific knowledge
They are witnesses to the vast changes of time
And marvelous creations of nature
That reason alone is enough

As human civilization entered the information age,
the world began to change too quickly.
Life became exhausting for many.
After a long day of work,
a person might find comfort in quietly gazing at a green stone called fluorite.
They may know it is calcium fluoride,
and that it plays a role in phones and toothpaste,
but in that moment, none of this knowledge matters.
All that matters is the quiet, soothing glow of the mineral.



Chapter Five: The Destiny of Minerals
In some mountainous regions,
extensive mining activity has taken place for decades,
leaving deep marks on the landscape.
Entire slopes have been turned into mines,
and the terrain remains steep and challenging.

Underground, the environment feels very different from the surface.
Some describe it as similar to being in outer space,
with the immense weight of rock overhead.
The tunnels stretch for kilometers,
linked by shafts and horizontal passages,
and traveling between levels can take a long time.
Although underground temperatures are cool,
the intensity of work often leaves miners drenched in sweat.
The excitement of uncovering valuable minerals drives the effort forward.

With lamps turned off and ultraviolet light switched on,
tiny blue specks sometimes appear on the rock walls.
These are signs of tungsten,
present in the form of scheelite embedded in mineral veins.
By following these veins,
ore-bearing rocks are carefully extracted.

The mined rocks are transported outward by small rail cars,
dumped, and then washed by workers leaning over guardrails.
Pieces rich in ore are quickly picked out,
while the rest is discarded.

Discarded rocks are often searched through by local people
hoping to find fragments of value.
Some individuals use geology hammers to break apart stones,
keeping promising samples and discarding the rest.
Selected pieces are crushed
and sent into sorting workshops along the slopes,
where the material is separated step by step.

Finally, on shaking tables,
the ore gradually concentrates.

The resulting black powder is tungsten.
This very hard metal is used in high-strength materials,
including tank armor.

Needle-like projectiles made of tungsten
demonstrate its unique properties in warfare,
able to pierce armor with astonishing force.

During mining, cavities are sometimes encountered,
locally referred to as “pockets.”
Inside, crystals may grow—
cubic, sheet-like, or in other striking forms.



The first impression of these crystals is often one of beauty,
followed by curiosity about how such forms can develop.

At the microscopic level,
the crystal lattice structure resembles
the geometric models studied in chemistry classes.
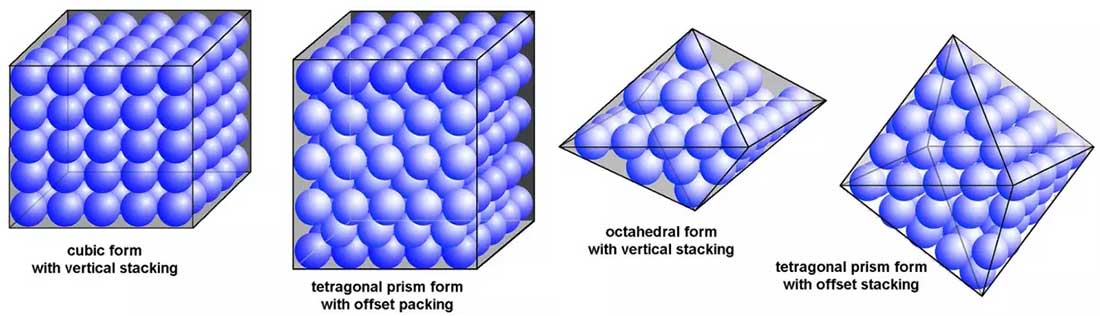
These mineral crystals are essentially
the natural realization of those lattice frameworks.

Not long ago, many fine crystals were smashed and discarded as waste.
Today, however, a single pocket of well-formed crystals
can be valued at millions.
Mineral specimens from such deposits
are now highly prized,
sometimes selling for tens or hundreds of thousands of dollars.
Collectors worldwide compete for the best pieces.
Fortunately, awareness of their scientific and aesthetic value has grown,
and many mines now preserve crystal specimens
rather than destroying them.
These minerals, once destined to become raw material for industry,
are increasingly appreciated as natural works of art
and displayed in museums and private collections.


When all is quiet at night,
gazing at these minerals
can feel like a silent conversation with the stars.
Though separated by vast distances and ages,
both humanity and these crystals
share a common origin in ancient stellar explosions.